Abstract
Leukemia relapse and treatment related mortality (TRM) remain major obstacles for successful allogeneic hematopoietic cell transplantation (allo-HCT). The number of induction cycles using intensive chemotherapy at AML diagnosis to achieve complete remission (CR) and the number of consolidation cycles and disease status at the time of allo-HCT for patients with acute myeloid leukemia (AML) may each affect TRM and relapse rates. We investigated the impact of the number of induction/consolidation cycles and disease status on the success of allo-HCT in 3113 AML patients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) (2008-2019). They received allo-HCT in first CR or with persistent leukemia (primary induction failure-PIF) receiving myeloablative (MA) or reduced-intensity (RIC) conditioning.
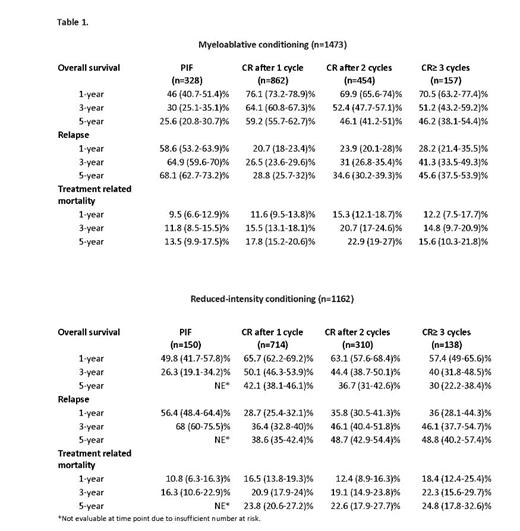
1473 AML patients (median age, 47 years) in CR received MAC; 862 (58%) achieved CR after 1 cycle of intensive induction chemotherapy and 74% of these had no evidence of measurable residual disease (MRD). 454 (31%) patients required 2 cycles to CR (72 % MRD negative) and 157 (11%) patients (69% MRD negative) after ≥ 3 cycles. The overall survival (OS), relapse and TRM by induction cycle number is shown in Table 1. Multivariate analysis demonstrated that CR after 1 cycle led to higher OS vs. 2 cycles (HR 1.32 95%CI 1.11-1.56, p< 0.01) or ≥ 3 cycles (HR 1.47 95%CI 1.16-1.87, p< 0.01), while OS after 2 cycles or ≥ 3 cycles were similar (HR 1.2 95%CI 0.87-1.4, p=0.38). Higher TRM was observed in patients receiving 2 or ≥ 3 cycles vs. only 1 induction cycles (HR 1.34 95%CI 1.05-1.72, p< 0.02). Relapse risk was greater in those needing ≥ 3 cycles to achieve CR. Consolidation therapy after CR was associated with improved OS vs. no consolidation therapy (HR 1.57 95%CI 1.24-1.99, p< 0.01). The need for ≥2 induction cycles plus consolidation therapy was associated with higher TRM (HR 1.34 95%CI 1.05-1.72, p< 0.02). 1162 AML patients (median age, 63 years) in CR received allo-HCT after RIC; 714 (61%) achieved CR after 1 cycle of induction chemotherapy (72% MRD negative); 310 (27%) patients after 2 cycles (67% MRD negative) and 138 (12%) patients (58% MRD negative) after ≥ 3 cycles (Table 1). Multivariate analysis demonstrated that the number of induction cycles did not affect the OS or TRM. Relapse risk was greater in patients requiring ≥2 cycles to achieve CR. The use of consolidation therapy did not affect OS or TRM. MRD status at the time of allo-HCT did not have a significant impact on OS, TRM and relapse rates after either MA or RIC conditioning.
478 AML patients received allo-HCT after PIF (328 patients with MAC [median age, 51 years], 150 patients RIC [median age, 61 years], Table 1). After MAC, OS and relapse were significantly worse in PIF patients compared to any CR patients (p<0.01). After RIC, relapse was significantly more frequent in PIF patients vs. CR patients after 1 or more induction cycles (p<0.01). TRM was similar for PIF vs CR patients after MAC or RIC allo-HCT.
These data demonstrate that among patients eligible for allo-HCT, the need for only one induction cycle to achieve CR, particularly when combined with consolidation therapy is associated with better outcomes after MA conditioning. Achieving CR prior to allo-HCT needing one or more induction cycles is associated with lower relapse rates and improved OS compared to patients with PIF that receive allo-HCT.
de Lima: BMS: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotec: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Hourigan: Govt. COI: Other. Litzow: Omeros: Other: Advisory Board; Pluristem: Research Funding; Jazz: Other: Advisory Board; AbbVie: Research Funding; Amgen: Research Funding; Actinium: Research Funding; Astellas: Research Funding; Biosight: Other: Data monitoring committee. Saber: Govt. COI: Other. Weisdorf: Incyte: Research Funding; Fate Therapeutics: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal